
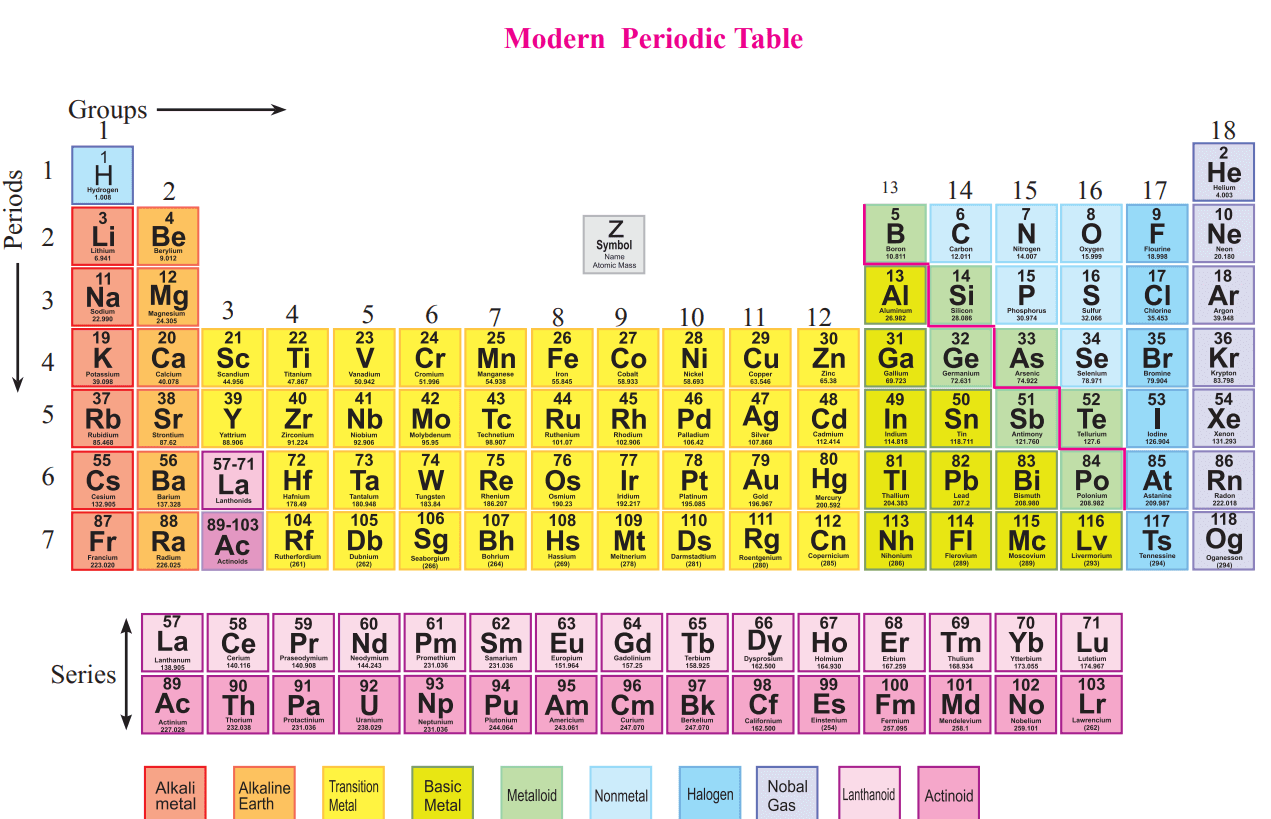
You will hear about, and why they react in very similar ways. Let's just think a little bit about some of the groups that Metals, in the D block, but we're not gonna go into those details. Of interesting exceptions that happen in the transition Outermost shell electrons, but there are exceptions to that, and there's actually a lot These are the electrons that are going to react, which tend to be the Number of valence electrons, and valence electrons andĮlectrons in the outermost shell, they tend to coincide, although, there's a slightly different variation. The elements in the column have very very very similar properties, and that's because theĮlements in a column, or the elements in a group, tend to have the same number of electrons in their outermost shell. There's tons of exceptions, but for the most part, But what's interesting, whyĭo we go through the trouble about calling one of these columns, of calling these columns a group? Well, this is what's interestingĪbout the periodic table, is that all of the elements in a column, for the most part, and Y'all might be thinking, what about these f-blockĮlements over here? If we were to properlyĭo the periodic table, we would shift all of these, everything from the d-blockĪnd p-block rightwards, and make room for these f-block elements, but the convention is is
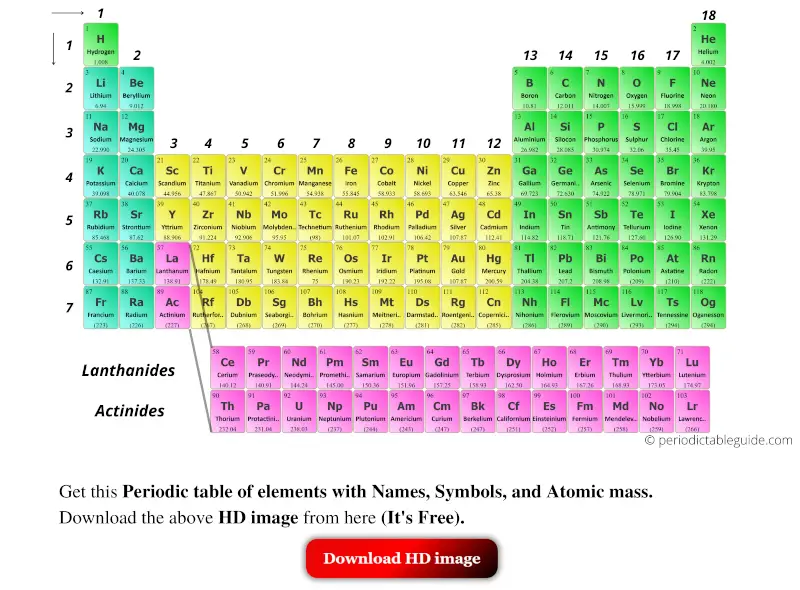
So that's group one, second column, third group,įourth, fifth, sixth, seventh, eighth, group To think about groups is that they just are theĬolumns of the periodic table, and standard convention is to number them. A little bit about groups of the periodic table.


 0 kommentar(er)
0 kommentar(er)
